
Back كينين Arabic کوینین AZB Хінін BE Хинин Bulgarian কুইনিন Bengali/Bangla Quinina Catalan Chinin Czech Cwinin CY Kinin Danish Chinin German
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | US: /ˈkwaɪnaɪn/, /kwɪˈniːn/ or UK: /ˈkwɪniːn/ KWIN-een |
| Trade names | Qualaquin, Quinbisul, others[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682322 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth, intramuscular, intravenous, rectal |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 70–95%[4] |
| Metabolism | Liver (mostly CYP3A4 and CYP2C19-mediated) |
| Elimination half-life | 8–14 hours (adults), 6–12 hours (children)[4] |
| Excretion | Kidney (20%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.004.550 |
| Chemical and physical data | |
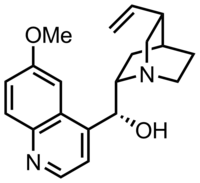
| Formula | C20H24N2O2 |
| Molar mass | 324.424 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 177 °C (351 °F) |
| |
| |
| | |
Quinine is a medication used to treat malaria and babesiosis.[5] This includes the treatment of malaria due to Plasmodium falciparum that is resistant to chloroquine when artesunate is not available.[5][6] While sometimes used for nocturnal leg cramps, quinine is not recommended for this purpose due to the risk of serious side effects.[5] It can be taken by mouth or intravenously.[5] Malaria resistance to quinine occurs in certain areas of the world.[5] Quinine is also used as an ingredient in tonic water to impart a bitter taste.[7]
Common side effects include headache, ringing in the ears, vision issues, and sweating.[5] More severe side effects include deafness, low blood platelets, and an irregular heartbeat.[5] Use can make one more prone to sunburn.[5] While it is unclear if use during pregnancy carries potential for fetal harm, treating malaria during pregnancy with quinine when appropriate is still recommended.[5] Quinine is an alkaloid, a naturally occurring chemical compound.[5] How it works as a medicine is not entirely clear.[5]
Quinine was first isolated in 1820 from the bark of a cinchona tree, which is native to Peru,[5][8][9] and its molecular formula was determined by Adolph Strecker in 1854.[10] The class of chemical compounds to which it belongs is thus called the cinchona alkaloids. Bark extracts had been used to treat malaria since at least 1632 and it was introduced to Spain as early as 1636 by Jesuit missionaries returning from the New World.[11] It is on the World Health Organization's List of Essential Medicines.[12][13] Treatment of malaria with quinine marks the first known use of a chemical compound to treat an infectious disease.[14]
- ^ "Quinine International". Drugs.com. 2 November 2020. Retrieved 8 November 2020.
- ^ a b "Quinine Use During Pregnancy". Drugs.com. 25 March 2020. Retrieved 13 August 2020.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ a b "Qualaquin (quinine) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Archived from the original on 2 February 2014. Retrieved 29 January 2014.
- ^ a b c d e f g h i j k l "Quinine sulfate". Drugs.com. 20 February 2020. Retrieved 14 May 2020.
- ^ Esu EB, Effa EE, Opie ON, Meremikwu MM (June 2019). "Artemether for severe malaria". The Cochrane Database of Systematic Reviews. 6 (6): CD010678. doi:10.1002/14651858.CD010678.pub3. PMC 6580442. PMID 31210357.
- ^ Olmsted J, Williams GM (1997). Chemistry: The Molecular Science. Jones & Bartlett Learning. p. 137. ISBN 978-0-815-18450-8. Archived from the original on 15 September 2016.
- ^ Willcox M (28 June 2004). Traditional Medicinal Plants and Malaria. CRC Press. p. 231. ISBN 9780203502327.
- ^ Cechinel-Filho V (2012). Plant bioactives and drug discovery : principles, practice, and perspectives. Hoboken, N.J.: John Wiley & Sons. p. 2. ISBN 9780470582268. Archived from the original on 4 March 2016.
- ^ Strecker A (1854). "Ueber einen neuen aus Aldehyd - Ammoniak und Blausäure entstehenden Körper". Liebigs Ann. Chem. 91 (3): 349–351. doi:10.1002/jlac.18540910309.
- ^ Staines HM, Krishna S (2011). Treatment and Prevention of Malaria : Antimalarial Drug Chemistry, Action and Use. [S.l.]: Springer Verlag. p. 45. ISBN 9783034604796.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- ^ "Quinine". Encyclopedia Britannica. Retrieved 12 November 2021.