
Back أوباداسيتينيب Arabic Upadacitinib Spanish Upadacitinib French אופדסיטיניב HE ウパダシチニブ Japanese ଉପାଡାସିଟିନିବ OR
 | |
| Clinical data | |
|---|---|
| Pronunciation | /juˌpædəˈsaɪtɪnɪb/ ew-PAD-ə-SY-ti-nib |
| Trade names | Rinvoq |
| Other names | ABT-494 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a619051 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Janus kinase (JAK) inhibitor |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 52% |
| Metabolism | Liver (CYP3A major, CYP2D6 minor)[13] |
| Metabolites | M4, an acyl glucuronide |
| Elimination half-life | 9–14[12] (6–15[13]) hours |
| Excretion | Mainly unchanged in feces (38%) and urine (24%)[12] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
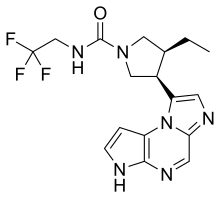
| Formula | C17H19F3N6O |
| Molar mass | 380.375 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Upadacitinib, sold under the brand name Rinvoq, is a medication used for the treatment of rheumatoid arthritis, psoriatic arthritis, atopic dermatitis, ulcerative colitis, Crohn's disease, ankylosing spondylitis, and axial spondyloarthritis.[10][11] Upadacitinib is a Janus kinase (JAK) inhibitor that works by blocking the action of enzymes called Janus kinases.[10][14][11] These enzymes are involved in setting up processes that lead to inflammation, and blocking their effect brings inflammation in the joints under control.[11]
Common side effects include upper respiratory tract infections (common cold, sinus infections), nausea, cough, and fever.[10][11]
Upadacitinib was approved for medical use in both the United States and the European Union in 2019.[11][14][15][16]
- ^ "Upadacitinib (Rinvoq) Use During Pregnancy". Drugs.com. 23 September 2019. Archived from the original on 18 March 2020. Retrieved 17 March 2020.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ "AusPAR: Upadacitinib". Therapeutic Goods Administration (TGA). 25 August 2021. Archived from the original on 4 September 2021. Retrieved 4 September 2021.
- ^ "Rinvoq (Abbvie Pty Ltd)". Archived from the original on 9 November 2022. Retrieved 9 November 2022.
- ^ "Rinvoq (Abbvie Pty Ltd)". Therapeutic Goods Administration (TGA). 16 February 2023. Archived from the original on 18 March 2023. Retrieved 9 April 2023.
- ^ "Rinvoq Product information". Health Canada. 25 April 2012. Archived from the original on 30 May 2022. Retrieved 29 May 2022.
- ^ "Summary Basis of Decision (SBD) for Rinvoq". Health Canada. 23 October 2014. Archived from the original on 31 May 2022. Retrieved 29 May 2022.
- ^ "Regulatory Decision Summary for Rinvoq". Drug and Health Products Portal. 21 July 2023. Retrieved 1 April 2024.
- ^ "Rinvoq 15 mg prolonged-release tablets - Summary of Product Characteristics (SmPC)". (emc). 1 March 2020. Archived from the original on 27 August 2021. Retrieved 22 August 2020.
- ^ a b c d Cite error: The named reference
Rinvoq FDA labelwas invoked but never defined (see the help page). - ^ a b c d e f "Rinvoq EPAR". European Medicines Agency (EMA). 16 October 2019. Archived from the original on 20 October 2020. Retrieved 29 April 2020.
- ^ a b Cite error: The named reference
AssessmentReportwas invoked but never defined (see the help page). - ^ a b Mohamed MF, Camp HS, Jiang P, Padley RJ, Asatryan A, Othman AA (December 2016). "Pharmacokinetics, Safety and Tolerability of ABT-494, a Novel Selective JAK 1 Inhibitor, in Healthy Volunteers and Participants with Rheumatoid Arthritis". Clinical Pharmacokinetics. 55 (12): 1547–1558. doi:10.1007/s40262-016-0419-y. PMID 27272171. S2CID 39036534.
- ^ a b "Drug Trials Snapshots: Rinvoq". U.S. Food and Drug Administration (FDA). 16 August 2019. Archived from the original on 5 August 2020. Retrieved 18 March 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ Cite error: The named reference
Drug Approval Package: Rinvoqwas invoked but never defined (see the help page). - ^ "AbbVie Receives FDA Approval of Rinvoq (upadacitinib), an Oral JAK Inhibitor for the Treatment of Moderate to Severe Rheumatoid Arthritis" (Press release). AbbVie. Archived from the original on 16 August 2019. Retrieved 16 August 2019.