
Back يوزين واي Arabic اوزین وای AZB Eosin Y German ائوزین وای FA Eozin Y Croatian 에오신 Y Korean Eosina Y Portuguese Эозин Н Russian Eozin Y SH Eozin Y Serbian
| |

| |
| Names | |
|---|---|
| IUPAC name
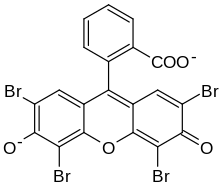
2-(2,4,5,7-tetrabromo-6-oxido-3-oxo-3H-xanthen-9-yl)benzoate [in its deprotonated form]
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.037.629 |
| MeSH | Eosine+Yellowish-(YS) |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C20H6Br4Na2O5 | |
| Molar mass | 647.89052 |
| Appearance | Red powder |
| Density | 1.018 g·cm−3 |
| Melting point | 295.5 °C (563.9 °F; 568.6 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Eosin Y, also called C.I. 45380[1][2] or C.I. Acid Red 87,[2][1] is a member of the triarylmethane dyes. It is produced from fluorescein by bromination.[3]
- ^ a b Cite error: The named reference
Bancroft and Stevens, 1982was invoked but never defined (see the help page). - ^ a b Cite error: The named reference
Lillie, 1977was invoked but never defined (see the help page). - ^ Gessner, Thomas; Mayer, Udo (2000). "Triarylmethane and Diarylmethane Dyes". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a27_179. ISBN 978-3527306732.